Pharmaceutical production
At Alfa Laval, we believe in creating better everyday conditions for the Pharma & Biotech industry, so that together, we can help even more people towards a healthier and happier life. Watch this video to learn more about how Alfa Laval can contribute to your pharmaceutical and biotech processes.
- Cost-efficient operations
- More uptime and better yields along with highest level of hygiene
- Safeguard product characteristics and quality with in-batch and batch-to-batch consistency
- Made for smooth commissioning, qualification and validation
- Compliant with relevant standards
With our equipment and the diversity of our global network of sales and service representatives, we provide sustainable solutions that meet the highest demands. We strive to improve customer competitiveness through better operational efficiency and high quality end-products backed by engineering experience, application knowledge, customized installation and full validation support.
Blood plasma processing
Securing highest purity, safety and yield
Blood plasma is an important starting material for many of our medicinal treatments. High quality and robust equipment securing gentle product treatment are essential for optimized blood plasma fractionation and processing. Our tried and tested portfolio, including fluid handling components, heat exchangers and membrane filtration, is engineered to maintain strict hygiene standards and deliver repeatable high-quality products.
Chemical API processing
Safe, efficient and simple solutions for complex products
Process efficiency is key to successful production of chemical active pharmaceutical ingredients (APIs) from complex chemical substances. Increasingly, drug companies look to Alfa Laval as a turnkey supplier of process expertise, hygienic equipment and services for the manufacture of high quality chemical APIs used in finished pharmaceutical products. Equipment from Alfa Laval meets regulatory requirements, supports product stability, lowers manufacturing costs and increases production yields.
Reliability, consistency, and regulatory compliance are vital to formulating and delivering effective pharmaceuticals. Alfa Laval has a deep understanding of pharmaceutical manufacturing, which we invest in our broad range of hygienic equipment designed to meet the stringent requirements of final formulation processing
.
Pharmaceutical water systems
Solutions for optimizing water for injection (WFI), purified water (PW), and non-compendial water systems
Water is an essential ingredient to all pharmaceutical processing. Securing consistent high-quality water, delivered to the point-of-use, at required flow and temperature are basic requirements. Alfa Laval equipment offers you hassle-free operation with minimized operating costs, downtime and environmental impact.
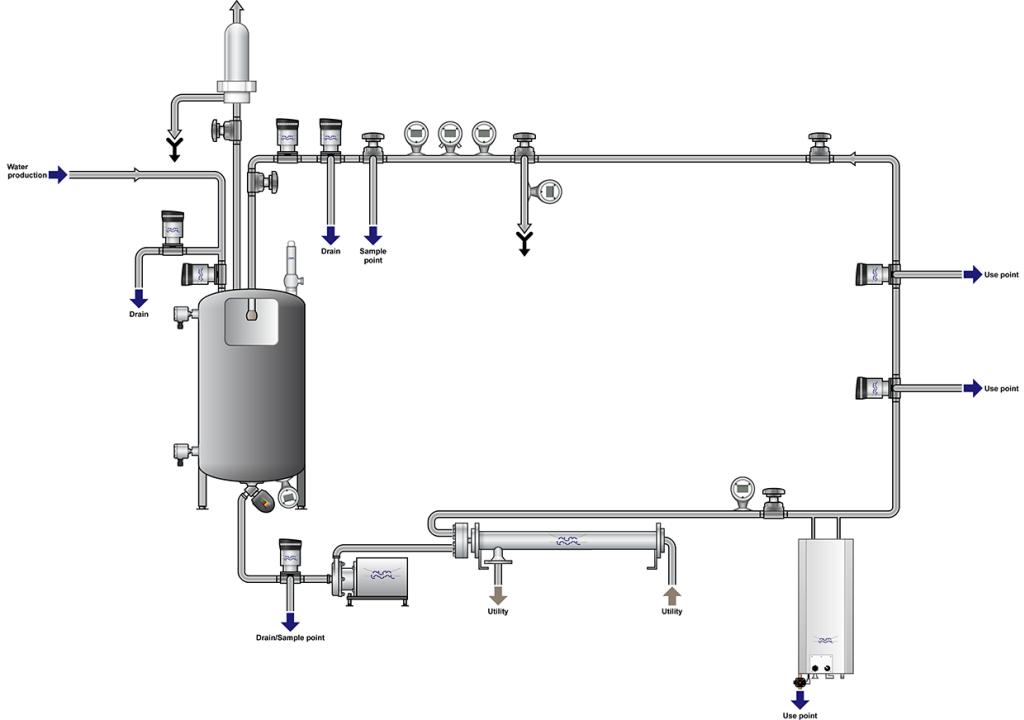
If you’re a pharmaceutical manufacturer looking for sustainable, energy-efficient solutions, or looking to prevent, rouging, biofilm buildup or other impurities in your pharmaceutical grade water system, Alfa Laval is here to help you meet stringent hygienic demands. With Alfa Laval, it is easy to comply with all the pharmaceutical requirements for safety, hygiene, economy, energy-efficiency and reduced waste. Take advantage of our vast pharmaceutical process expertise, broad and proven hygienic equipment and global service network.
Bacterial and fungus processing
Your equipment and solution provider for optimized fermentation processes
Improved yield, safety, quality and cost of operations are vital for staying competitive. Whether you manufacture insulin, vaccines, antiviral medicines or other therapeutics via bacterial or fungus processing, Alfa Laval provides an extensive range of proven separation solutions, fluid handling components and heat exchangers specifically designed to optimize your fermentation processes.
Tap on our years of experience in preparation of bacterial and fungus cultures to secure high quality end-products at highest yields
- Engineering experience and application expertise to optimize your bioprocesses
- Gentle product treatment to secure highest yields
- Cost-efficient operations – less waste handling and lower utilities consumption
- Safeguard product characteristics and securing consistent quality
- Extensive range of equipment and solutions for efficient bioprocessing
With our range of equipment and global network of sales and service representatives, we provide sustainable solutions that meet the highest demands. Backed by engineering experience, application knowledge, customized installation and full validation support, we aim to help customers improve competitiveness through better operational efficiency and maintaining high quality of end-products.
Plant and animal extract production
Maximize yields and ensure high product quality
Natural products provide many viable extracts for the pharmaceutical industries. Maintaining their remedial properties during extraction is key to the success and commercialization of these products. Alfa Laval’s years of experience and proven processing equipment for extraction, separation, purification and concentration enable you to achieve high product quality and top recovery levels in a hygienic and low-energy environment.
Cell culture processing
Ultra-pure equipment and gentle product treatment to optimize your biopharmaceutical processes
For production of high quality medicines, Alfa Laval offers an extensive range of separation solutions, fluid handling components and heat exchangers to optimize your processes, achieve higher yields or improve cost-efficiency, while fulfilling high standards of hygiene and reliability.
Offering years of experience in cell culture preparation and regulatory-compliant equipment
- Safeguard product characteristics and quality with in-batch and batch-to-batch consistency
- Higher yields and more uptime along with highest level of hygiene
- Made for smooth commissioning, qualification and validation
- Efficient operations – less waste handling and utilities consumption
- Compliant with relevant pharmaceutical standard
With our range of equipment and global network of sales and service representatives, we provide sustainable solutions that meet the highest demands in cell culture. Backed by complete documentation, full engineering and validation support, our innovative equipment offers operational efficiency and helps to maintain product integrity and improve competitiveness.
Egg-based vaccine production
Flexibility with safe and automated processes makes you ready for an influenza pandemic
To be prepared for a possible outbreak of an infectious disease is crucial for our world’s health and wellbeing. The need for fast acting with safe, robust and flexible process is a must to save our world from potential pandemics. Alfa Laval provides equipment and global support to help solve possible problems and to secure safe and fast manufacturing of egg-based influenza vaccines.
Industrial fermentation processes
From lab to full-scale, high capacity production of high-quality bio-based products with environmentally friendly processes
Years of experience in the growth, harvesting and purification of bio-based products
- High capacity processing with high yield and output
- Safeguard product characteristics with in-batch and batch-to-batch consistency
- Efficient operations with more uptime, less waste and lower utilities consumption
- Highest level of hygiene
- Compliant with relevant standards
With our range of equipment and global network of sales and service representatives, we provide sustainable solutions that meet the highest demands in bio-based product processing. The high capacity, operational efficiency and unique features of our equipment, backed by complete documentation, full engineering and validation support, helps to maintain product integrity, process output and improve your competitiveness.
Support and utility processes
Maintaining uptime in biotech and pharmaceutical industries
Whether it is CIP (Cleaning in Place), clean steam, waste treatment or kill system, Alfa Laval has an extensive range of equipment to keep your systems safe and hygienic all specially designed to maintain uptime and meet the stringent hygiene requirements of the biotech and pharma industries.
Bio-based chemicals
The biotech revolution is influencing the chemical industry by making it possible to economically produce a vast array of products hitherto sourced from fossil raw materials or chemical synthesis. These chemicals are based on the fermentation of renewable resources with a low environmental impact and without requiring hazardous and often toxic production steps
Tube & Fitting
Tri-Clover UltraPure ASME BPE fittings
Fittings for pharmaceutical and biotech applications
Alfa Laval Tri-Clover UltraPure ASME BPE range includes a comprehensive selection of tubes and fittings of varying dimensions and executions all compliant with ASME BPE standards. Made for safe and easy installation, its high quality and good weldability meet the standards of biopharmaceutical industries.

- Very low contamination risk – consistent, easy to clean internal surface finishes (electro or mechanically polished) with Ra of < 0.4 - 0.8μ
- Easy and safe installation – secure, self-aligning joints and smooth, crevice-free, corrosion-resistant interiors for guaranteed hygiene
- Reliability minimizes risk – incoming and outgoing raw material/product quality control and close monitoring of manufacturing process
- Integrity secured – tubes from the same material supplier ensure uniformity, high quality welds and assured chemical composition and passivity state
- Versatile – selection of tubes, bends, unions, clamps, reducers available in a range of surface finishes and dimensions
Optimize your processes and maximize uptime with Alfa Laval Tri-Clover UltraPure ASME BPE. Get fast access to replacement parts and service, readily available through our local offices and more than 1500 sales and working partners around the world. Whether you require single fittings, boxes, pallets or even containers directly from the
Inline Valve
Unique DV-ST UltraPure
Diaphragm valve for a range of duties including dosing, filling, diverting and controlling in aseptic or hygienic systems
Alfa Laval Unique DV-ST UltraPure diaphragm valves are the natural choice for pharma and biotech industries (as well as other hygiene-focused processes). The compact and lightweight valves comprise a series of base parts including valve bodies, diaphragms, actuators and handles. The choice gives you a multitude of configurations to meet the exact needs of your process. All diaphragms used meet FDA/USP classifications as well as TSE/ADI.

High-quality valve bodies and wide range of proven diaphragms
- Reliable, long life diaphragm – available in high performance EPDM for continuous steam operation at temperatures up to 150 °C
- Reduced risk of contamination - fully documented, made for aseptic operation with standard low-ferrite valve body (option for high alloy solution)
- Simple and reliable stainless steel actuator with 5-year warranty – one-type-fits-all design makes selection safe, fast and easy
- Hygienic design – high-grade valve bodies and low Ra surface finishes
- Easy installation/validation/qualification and backed by Alfa Laval Q-doc comprehensive documentation package and service partner network
The actuator operates at up to 10 and 6 bar depending on diaphragm material (EPDM and PTFE/EPDM: 10 bar; TFM/PTFE: 6 bar). Autoclavability and chemical resistance ensure long service life and uptime. Over-closure protection (manual version) also helps prolong diaphragm life. All steel parts are made of stainless steel or composite material depending on handle type. There is also a range of easily mounted indication and control units
Unique Mixproof UltraPure
One valve providing double block and bleed with simplicity, safety and efficiency in mind - designed for the Pharmaceutical industry
Alfa Laval Unique Mixproof UltraPure is designed to suit the needs of the Pharmaceutical and Biotech industries. It enables the simultaneous flow of two different fluids without the risk of cross-contamination. Modular in design, they ensure maximum uptime for continuous, high-purity processes.

The high-purity, double seat valve for continuous processes

Aseptic and sterilizable solution for safe sample collection
- Design ensures hygienic and contamination-free sampling
- Easy to operate thanks to simple working principle
- Highly reliable operation (rated to 10 bar) and three year warranty for all non-wearing parts
- Safe sampling through features such as non-return, quick couplings, pressure relief valve for steam and more
- Enhanced cleanability - valve body made of single piece of stainless steel provides smooth, crevice-free surfaces eliminating bacterial build-up risk
Certification, specification and standards: All Unique Sampling Valves are designed, tested and approved according to EHEDG guidelines and certified to carry the 3-A symbol. All product-wetted components are made of 1.4404 (316L). EN10204 3.1 included with valve.
Mixing Technology
LeviMag® UltraPure
The aseptic magnetic mixer for mixing down to the last drop in sterile process applications. Superior hygiene, optimized operation
The Alfa Laval LeviMag® UltraPure is an aseptic magnetic mixer that uses a patented levitating impeller and advanced design to mix down to the last drop and maximize product yield. Compact, energy-efficient and easy to maintain, it is perfect for biotech, pharmaceutical and demanding hygienic and sterile applications such as those involving serums, vaccines, plasma fractions, bacteria and cell cultures, and APIs.

- Optimized flow for higher efficiency and more gentle product treatment
- Mixing down to the last drop for maximum yield due to low agitation and dry-running capability
- Optimized Cleaning-in-Place thanks to full drainability
- Minimized downtime due to ease of maintenance
- Comprehensive documentation for full traceability of the entire supply chain
Maximize product yield and quickly realize return on investment due to the dry-running capability of the Alfa Laval LeviMag UltraPure magnetic mixer. Ensuring gentle product treatment is easy due to efficient mixing at very low speeds. The open design and low-speed rotation during cleaning contribute to no dead zones, effective residue removal and minimize contamination risks from wear particles
ALB
Bottom-mounted agitator for hygienic low maintenance mixing and blending in food, dairy, beverage and pharmaceutical applications
The Alfa Laval ALB bottom-mounted agitator is suitable for atmospheric and pressurized tanks and comes in a range of sizes. The modular design and choice of size ensures an agitator fit-for-purpose so you won’t have to invest more than needed and can look forward to optimal power consumption. The ALB agitators are designed for easy CIP and can also be for use in sterile/aseptic and ATEX-certified applications.
Excellent levels of hygiene for a wide range of cost-effective, tank-based blending and mixing duties

- Easily tailored to meet specific tank/process requirements - modular design with hygienic materials increases flexibility and optimizes operation
- Reduced energy consumption – unique impeller design and product heat absorption makes ALB up to 400% more efficient than impellers with standard pitch
- Hygienic design and smooth surfaces meet EHEDG, USDA, FDA and 3-A standards
- Operates at low speeds without reducing pumping capacity – up to 80% less power consumption compared with conventional impellers
- High productivity – low wear and low maintenance (no dismantling or tank entry, for instance, to replace long-life seals and bearings)
We have versions for sterile/aseptic applications and optional equipment, including welding flange, blind flange, cover for motor/gear and spare part kit, for all ALB bottom-mounted agitators. ATEX certification and material certificates are available on request
ALT
Top-mounted agitator with free-hanging shaft for hygienic, low maintenance mixing and blending in food, dairy and pharmaceutical applications
The Alfa Laval ALT top-mounted agitator is suitable for atmospheric and pressurized tanks and comes in a range of sizes. The modular design and sizing options means you won’t have to invest more than needed and can look forward to optimal power consumption. ALT agitators are designed for easy CIP and can also be configured for use in sterile/aseptic and ATEX-certified applications.

Excellent levels of hygiene for a wide range of cost-effective, tank-based blending and mixing duties
- Easily tailored to meet specific tank/process requirements – modular design with hygienic materials increases flexibility and optimizes operation
- Reduced energy consumption – unique impeller design and product heat absorption makes ALT up to 400% more efficient than impellers with standard pitch
- Hygienic design and smooth surfaces meet EHEDG, USDA, FDA and 3-A standards
- Operates at low speeds without reducing pumping capacity – up to 80% less power consumption compared with conventional impellers
- High productivity – low wear and low maintenance (For instance, no dismantling or tank entry required to replace long-life seals and bearings)
We offer versions for sterile/aseptic applications and optional equipment, including welding flange, blind flange, cover for motor/gear and spare part kit, for all ALT top-mounted free-hanging agitators. ATEX certification and material certificates are
Hybrid Powder Mixer
Ideal for the dairy, food and beverage industries, this cost-effective dual-stage inline powder dissolution unit quickly mixes and pumps wet and dry ingredients into a homogeneous blend.

Innovative inline powder-liquid mixer
The Alfa Laval Hybrid Powder Mixer is a patented hygienic mobile and stationery unit that uses a single standard motor to disperse powders into liquids quickly and efficiently, and to pump the combined solution. By combining pump and powder dissolving technologies, this versatile, easy-to-use mixer produces homogeneous products at high productivity while delivering significant energy savings.
Designed for batch production, it is the only inline powder mixer with a single motor drive that is able to disperse solids into liquids uniformly while simultaneously pumping the process liquid at pressures of up to 5 bar. It is an excellent choice for use in a wide variety of dairy, food and beverage powder mixing applications, such as dissolving stabilizers like pectin and xanthan gum and emulsifiers in the percentages required for most applications. It is also capable of producing recombined milk with more than 50% dry matter.
In addition, when used in combination with an Alfa Laval Rotary jet mixer, the Hybrid Powder Mixer may also be used as part of an efficient cleaning-in-place (CIP) system
Pump
LKH UltraPure
Centrifugal pumps designed to meet the specific demands of pharmaceutical and biotech applications
Alfa LKH UltraPure pharmaceutical pumps (certified to EHEDG) use a combination of optimised inlets and advanced impeller design to give unobstructed product flow, very low NPSH requirements and excellent hydraulic efficiency to keep your process running smoothly. They are designed for CIP and available in capacities of up to 500 m3/h and pressures of up to 190 m (19 bar).

Highly efficient and economical centrifugal pump that meets the requirements of hygienic processes
- Low contamination risk – hygienic design with pharma-grade SiC+ seals, pre-defined compression elastomers and low-delta ferrite impeller
- Maximized uptime and reduced maintenance costs – robust mechanical design and ease of maintenance with modular front-loading seals
- Increased yield – comes with full material traceability and USP Class VI elastomers to reduce risk of process contamination from extractables
- Energy and cleaning efficient – precise hydraulic design energy costs and CO2/waste chemical emissions to boost yields and process sustainability
- Smooth qualification, validation and process control – material traceability, and pump supplied with Alfa Laval’s Q-Doc package in line with GDP
The vane design of the semi-open impeller ensures a low Net Positive Suction Head required and prevents the effects of cavitation. The impeller balance holes help circulate the process fluid in the shaft seal area reducing axial force and wear on the seal and motor bearings. The high flow around the pump, especially behind the impeller near the shaft seal, makes cleaning easy and enhances the lubrication/ cooling of the seal to prolong life
Documentation
Alfa Laval Q-doc documentation package
Alfa Laval Q-doc is our comprehensive documentation package for our UltraPure equipment. Based on Good Documentation Practice (GDP), Q-doc documents every aspect from raw material to delivered equipment. With full transparency of sourcing, production and supply chains it is a simple matter to trace even the slightest change in material or manufacturing procedures – even when it comes to spare parts.
Documentation for biotech and pharmaceutical needs
The standard Alfa Laval Q-doc documentation package for Alfa Laval UltraPure equipment ensures full traceability of all product contact parts such as, steel, gaskets, etc. This secures a perfect match every time and prevents potential oversights that could necessitate revalidation.
Alfa Laval Q-doc comprises conformity declaration, material certificates, relevant test certificates and information about necessary Alfa Laval spare parts kits for standard components. Just like the factory acceptance tests (FATs) for our separation systems, the Q-doc documentation package supports a smooth qualification and validation process, and safeguards long-term peace of mind.
Downloading Q-doc documentation package
Want to locate an Alfa Laval Q-doc?
Customers can now download Alfa Laval Q-doc here: www.alfalaval.com/qdoc


